Dual Nature of Radiation and Matter
NCERT Chapter 11 • Photoelectric Effect & de Broglie Wavelength
1. Electron Emission
Free electrons in metals require minimum energy to escape the surface. This energy can be supplied via:
- Thermionic Emission: By heating the metal (e.g., filament in vacuum tubes).
- Field Emission: By applying a strong electric field (~10⁸ V/m).
- Photoelectric Emission: By irradiating with light of suitable frequency.
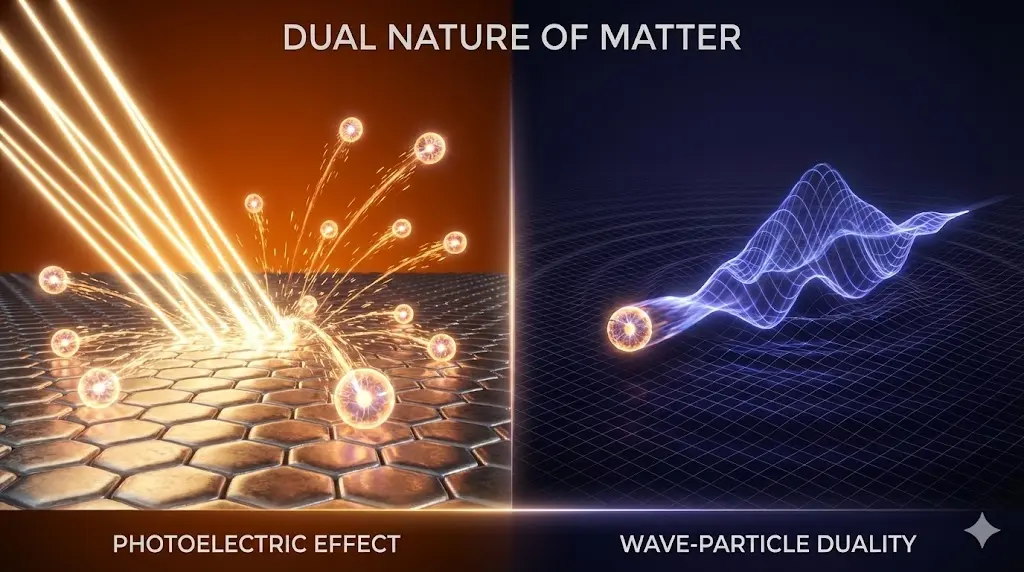
2. Photoelectric Effect
Discovered by Hertz (1887) and studied in detail by Hallwachs & Lenard.

- Work Function (
 ): Minimum energy to eject an electron (unit: eV).
): Minimum energy to eject an electron (unit: eV). - Threshold Frequency (
 ): Minimum frequency for photo-emission:
): Minimum frequency for photo-emission:  .
. - Photoelectrons: Electrons emitted due to light incidence.
3. Experimental Study
Key observations from Lenard’s experiments:
- Photocurrent ∝ Intensity (for
 ).
). - Stopping potential
 independent of intensity.
independent of intensity.  increases linearly with frequency
increases linearly with frequency  .
.- No emission if
 (even for high intensity).
(even for high intensity). - Emission is instantaneous (< 10⁻⁹ s).
A. Effect of Intensity

 Intensity (if
Intensity (if  ).
).B. Effect of Potential & Frequency

 ) to stop the electrons.
) to stop the electrons.4. Why Wave Theory Failed
- KE should increase with intensity (but it depends only on
 ).
). - No threshold frequency should exist (but
 is observed).
is observed). - Time lag for emission at low intensity (but emission is instantaneous).
5. Einstein’s Photoelectric Equation
Albert Einstein (1905) explained the effect using light quanta (photons). Awarded Nobel Prize in 1921.

Energy Conservation:
Photon energy = Work function + Max KE of electron.
Since
For
From ![]() , the slope is
, the slope is ![]() . Millikan verified this experimentally.
. Millikan verified this experimentally.

The graph of ![]() vs
vs ![]() is plotted for a single photosensitive material.
Different metals give different parallel lines (same slope
is plotted for a single photosensitive material.
Different metals give different parallel lines (same slope ![]() , different threshold frequencies
, different threshold frequencies ![]() ).
The x-intercept gives
).
The x-intercept gives ![]() , which is unique to each metal.
, which is unique to each metal.
From Einstein’s equation:
⇒
- Slope =
 → used to experimentally determine Planck’s constant.
→ used to experimentally determine Planck’s constant. - X-intercept = threshold frequency
 .
. - Y-intercept =
 → depends on work function of the material.
→ depends on work function of the material. - All metals give parallel lines (same slope, different intercepts).
6. Particle Nature of Light: Photons
- Energy:

- Momentum:

- Speed:
 (in vacuum)
(in vacuum) - Electrically neutral (not deflected by E/B fields)
- In collisions: Energy & momentum conserved, but photon number may change (absorption/emission).
7. Wave Nature of Matter
Louis de Broglie (1924) proposed that moving particles exhibit wave-like behavior.
de Broglie hypothesized this applies to all matter.
Momentum
8. Davisson-Germer Experiment
First experimental proof of matter waves (1927).

- Electron beam accelerated by voltage
 directed at nickel crystal.
directed at nickel crystal. - Scattered electrons detected at various angles.
- Peak intensity observed at specific angle (e.g., 50° for 54V).
- Bragg’s law applied:
 .
. - Calculated
 matched de Broglie’s formula.
matched de Broglie’s formula.
Solve numericals on Work Function and de Broglie Wavelength: Chapter 11 Important Questions →